

Our law firm has a solid reputation and a successful track record of helping accident victims collect the compensation they deserve.Ĭall Nash & Franciskato at (877) 284-6600. One of our experienced staff will speak with you personally and will provide you with a free, no-obligation review of your case.

If you are suffering adverse effects from the use of a defective medical device, contact our legal team in Kansas City today for your free, no-obligation case evaluation. Your email address will only be used to send you our newsletter and respond to inquiries. If you would like to receive news and blog updates on a regular basis, sign up to receive our email newsletter. The device allows health care providers access to a patient’s peripheral vascular system, for short term use, to sample blood, monitor blood pressure, or administer fluids, blood, and blood products. Product Codes: See Recall Database Entry.Product Name: ARROW Endurance Extended Dwell Peripheral Catheter System.The FDA defines these recalls as having a “reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.” Product Recall ClassificationĬlass I recalls are the most urgent because they have the potential to be the most harmful. Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX. As of today’s date, no litigation is pending this is strictly an alert to the general public. Blockage of the lung arteries (pulmonary embolism)Īccording to the release from Teleflex dated June 26, 2023, Teleflex/Arrow International reports 83 complaints related to this issue, 18 reported injuries and no deaths.The problem: If the catheter separates while in a blood vessel, the catheter fragments could be left in the bloodstream, and may migrate to other places in the body causing serious injury including: The FDA has classified this as a Class I recall, the most serious type of recall. 83 complaints have been filed that the device can separate or leak when in a patient’s blood vessel. Teleflex and its subsidiary Arrow International initiated a product recall of 262,016 Arrow Endurance Extended Dwell Peripheral Catheter systems on May 19, 2023. U.S.Teleflex Recalls Arrow Endurance Peripheral Catheter System.Data supports portfolio of HeartFlow precision coronary care solutions.
Endurance catheter portable#
Henry Schein, Medpod collaborate to launch Medpac portable telediagnostics system.Anteris Technologies touts first successful implant of DurAVR THV in valve-in-valve procedure.Inspire Medical appoints former AppliedVR exec as chief medical officer.Augmedics to acquire digital health assets from Surgalign auction.Abbott is pulling its Trifecta valves off the market.Baxter warns on some infusion pumps due to potential false alarms.Integra Lifesciences expects to resume manufacturing in Boston.Court rejects Johnson & Johnson bankruptcy gambit over talc suits.Tivic Health reduces workforce as it may face Nasdaq delisting.The company’s letter to customers instructs them to check their inventory for products within the recall’s scope - and stop using and quarantine the affected products.įiled Under: Catheters, Food & Drug Administration (FDA), News Well, Recalls, Regulatory/Compliance Tagged With: Teleflex

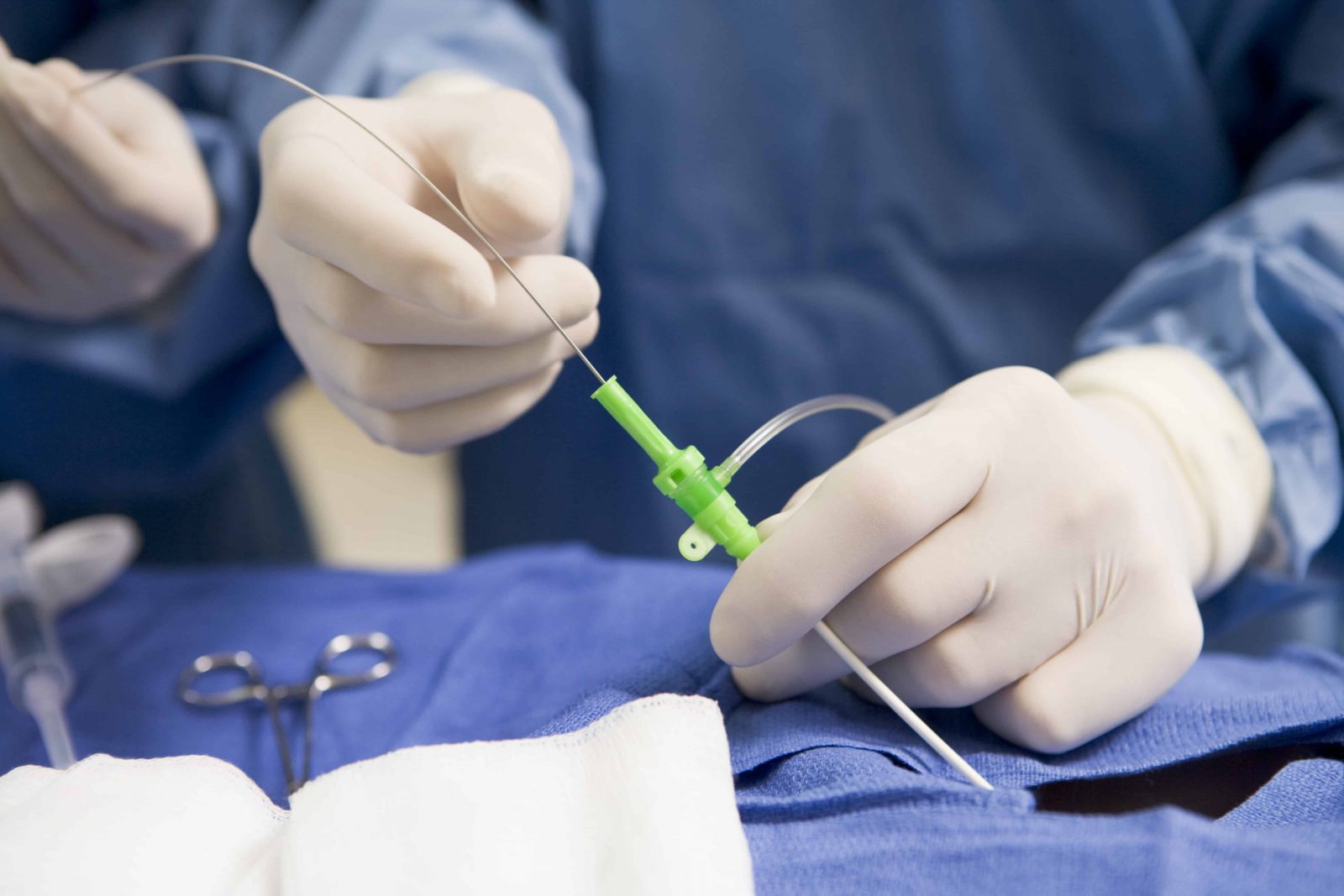
The situation could cause a person to experience a blockage of blood vessels, inadequate blood flow, injury to blood vessel walls, blood clots, blockage of the lung arteries (pulmonary embolism), heart attack, or death, according to the FDA. If the catheter separates while in a blood vessel, the catheter fragments could be left in the bloodstream and may migrate to other places in the body. Teleflex initiated a recall on May 19 after receiving reports of catheter separation or leakage. The Endurance system provides short-term access to a person’s peripheral vascular system, enabling health providers to sample blood, monitor blood pressure, or administer fluids, blood, and blood products. There are reports of 18 injuries but no deaths. Teleflex and its Arrow International subsidiary have reported 83 complaints related to this issue. The recall involved 262,016 devices distributed from October 26, 2018, to May 10, 2023, according to the FDA. The FDA today designated Teleflex’s recall of its Arrow Endurance extended dwell peripheral catheter system as Class I, the most serious type of medical device recall.


 0 kommentar(er)
0 kommentar(er)
